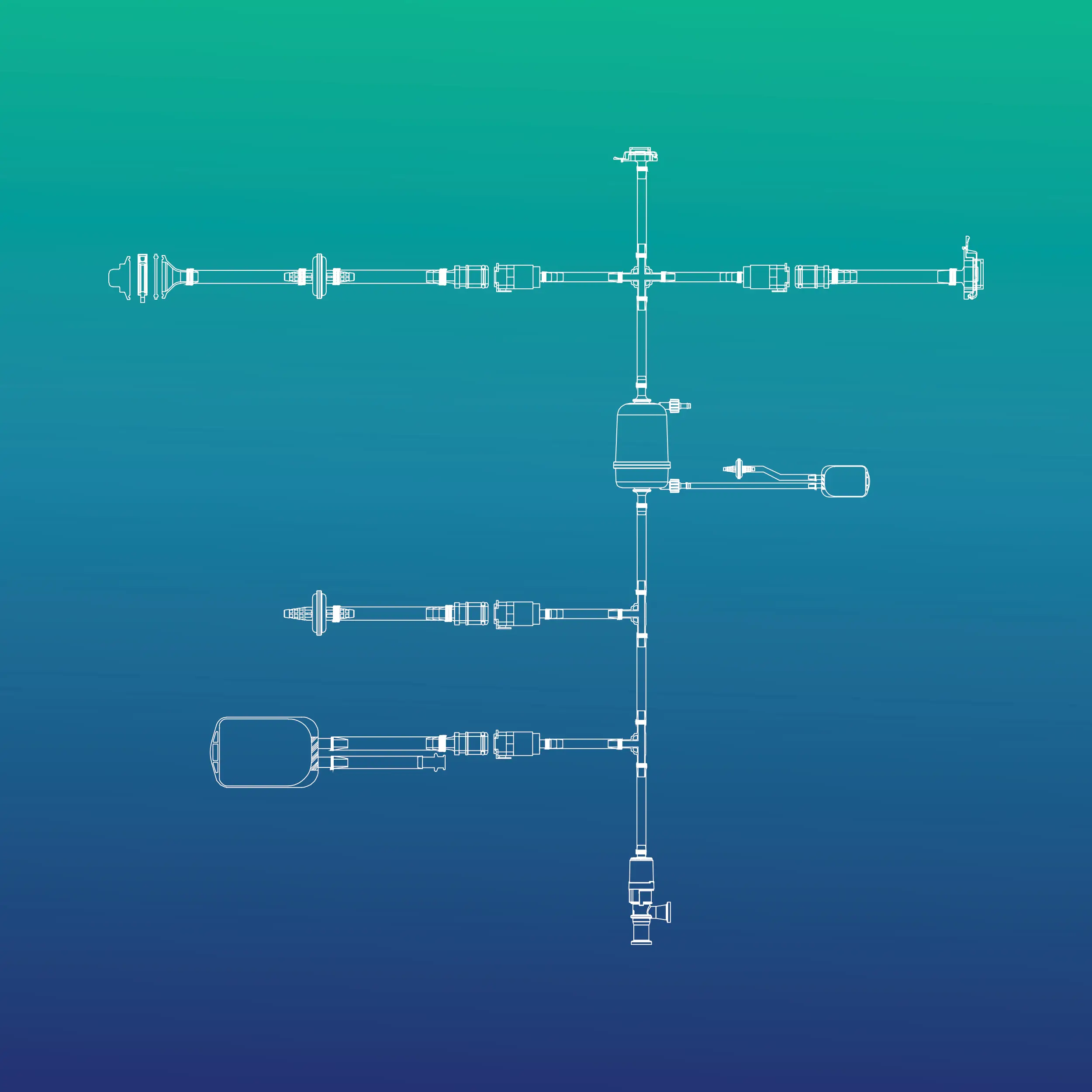
The PUPSIT assembly (Pre-Use Post-Sterilization Integrity Testing) is a critical component in the biopharmaceutical industry, designed to ensure the integrity of sterile filters before use. It involves testing filters after sterilization but prior to product filtration, ensuring no damage occurred during the sterilization process. This testing is mandatory in certain applications, particularly for sterile drug production, to guarantee that no contaminants pass through the filter during critical processes. The PUPSIT assembly includes connections to filters, sterile tubing, and instruments for performing integrity tests. It helps maintain regulatory compliance with guidelines like those from the EMA (European Medicines Agency). By verifying the filter's performance post-sterilization, PUPSIT ensures that the final drug product remains sterile and safe for use, minimizing contamination risks.